CBS and MTHFR deficiencies aren’t just rare childhood disorders—they may be hiding behind your adult patient’s “idiopathic” neurological symptoms.
We often associate inborn errors of metabolism with paediatrics: neonates in NICUs, metabolic crises, urgent interventions. But what happens when these genetic disorders masquerade as psychiatric illness, unexplained strokes, or progressive dementia in adults?
A 2007 paper in Revue Neurologique (Paris) presents a sobering reminder: cystathionine β-synthase (CBS) and methylenetetrahydrofolate reductase (MTHFR) deficiencies can present well into adulthood—sometimes catastrophically—but remain treatable if recognised in time. The real tragedy isn’t the genetics. It’s the diagnostic delay.
Let’s explore what happens when two key enzymes in homocysteine metabolism malfunction—and how your patient’s depression or stroke may have been preventable with methyl donors and betaine.
The Fork in the Pathway
Homocysteine sits at the junction of two essential routes:
- Transsulfuration: Homocysteine → cystathionine → cysteine (CBS-dependent; B6 required)
- Remethylation: Homocysteine → methionine → SAMe (MTHFR-dependent; folate, B12, betaine involved)
Disruption in either pathway leads to hyperhomocysteinemia, a toxic buildup with vascular, neurological, and psychiatric consequences.
CBS deficiency blocks the escape route through transsulfuration. MTHFR deficiency jams the recycling loop back to methionine. Both leave homocysteine stranded—and dangerous.
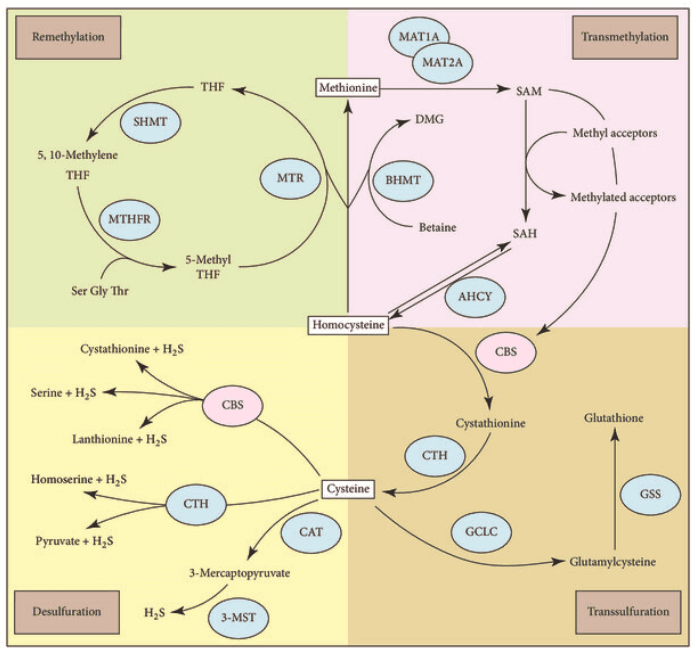

Image Source:
The Vascular and Neurological Fallout
CBS deficiency (classic homocystinuria) is notorious in the paediatric setting. But many patients aren’t diagnosed until adulthood, presenting instead with:
- Lens dislocation
- Osteoporosis
- Marfanoid habitus
- Thrombosis (arterial and venous)
- Neurological deterioration or psychiatric symptoms
By contrast, adult-onset MTHFR deficiency may appear silently until:
- Spinal cord degeneration
- Progressive cognitive decline
- Psychosis
- Stroke
- Coma
One case in the study involved a 56-year-old woman with years of misdiagnosed psychosis who eventually collapsed into coma. Her MRI showed white matter disease; her labs, homocysteine levels near 200 μmol/L. The cause? MTHFR deficiency. Within three months of betaine, B12, and folinic acid, she awoke—but remained paraplegic. A preventable outcome, had her homocysteine been tested earlier.
Lessons in Diagnosis
Here’s what makes this paper so clinically powerful: these disorders are not rare in function—they are rarely diagnosed.
A few critical flags:
- Homocysteine >100 μmol/L → think inborn error.
- Elevated homocysteine + elevated methionine → suspect CBS deficiency.
- Elevated homocysteine + low methionine → suspect MTHFR deficiency.
- Low folate in blood and CSF but normal methylmalonic acid? Think MTHFR.
Why does this matter? Because the treatment is relatively straightforward:
- CBS deficiency: Vitamin B6 (if responsive), folate, betaine, methionine-restricted diet.
- MTHFR deficiency: Betaine + B12 + folinic acid. Sometimes riboflavin.
Early treatment = prevention of irreversible neurological damage.
The Biochemistry of Symptoms
The effects of disrupted methylation and sulfur pathways go far beyond homocysteine levels.
- CBS deficiency causes homocysteine to flood the bloodstream, damaging vascular endothelium, triggering clot formation, and sometimes inducing seizures.
- MTHFR deficiency starves the methylation cycle, reducing SAMe—the universal methyl donor—crippling myelin synthesis, neurotransmitter production (serotonin, dopamine), and gene regulation. This translates into depression, psychosis, and neurodegeneration.
A simple nutrient gap can spiral into cognitive collapse.
Clinical Implications
If you’re a practitioner seeing:
- Young stroke
- Resistant psychiatric illness
- “Idiopathic” white matter changes
- Unexplained peripheral neuropathy
- Or early dementia with no vascular risk factors
You should consider ordering a plasma homocysteine test—ideally fasting—and assess methionine and methylmalonic acid to differentiate between B12 deficiency, CBS, and MTHFR defects.
Because once irreversible neurodegeneration sets in, it’s too late to backtrack.
What About the SNPs? Functional Genomics and the Grey Zone
While this paper focuses on overt, often devastating, biallelic mutations, most patients in functional medicine won’t have full-blown enzyme deficiencies. Instead, they’ll present with SNPs—common genetic variants that subtly alter enzyme activity and cofactor dynamics.
MTHFR C677T and A1298C
- C677T (especially TT genotype) can reduce enzyme function by up to 70%, impairing methylation and elevating homocysteine under stress.
- A1298C affects a different part of the enzyme and is more associated with neurotransmitter and BH4 imbalances than with homocysteine elevation.
In these patients, the symptoms are rarely overt but can manifest under pressure:
- Fatigue
- Anxiety or panic
- Histamine intolerance
- Hormonal dysregulation
- Subfertility
- Poor recovery from stress
CBS SNPs
Interestingly, CBS SNPs (e.g. rs234706, rs2851391) are often gain-of-function mutations. They may accelerate the transsulfuration pathway, pushing homocysteine quickly into cysteine or taurine. This sounds beneficial—until you consider the consequences:
- Loss of methyl groups upstream
- Ammonia and sulfite accumulation
- Impaired BH4 recycling
- Poor tolerance to methyl donors or high-protein diets
Clinically, this can look like:
- Agitation or brain fog with B vitamins, especially B6
- High sensitivity to sulfur-rich foods or supplements
- Oxidative stress with low glutathione reserves
- Chronic fatigue or inflammatory symptoms
Personalised Genomics in Practice
These patients don’t need the same protocols as those with overt disease—but they do need tailored support:
- MTHFR SNPs → Provide methylfolate, B12, riboflavin, choline.
- CBS SNPs → Balance methyl donors, support sulfur clearance, assess ammonia, and use sulfur cautiously.
- Monitor: Homocysteine, SAM:SAH ratio, and clinical response—not just genetic test results.
Understanding SNPs allows us to shift from reactive to proactive medicine—identifying and correcting imbalances before they trigger clinical disease.
It’s Not All or Nothing
Too often, we interpret genetics through a binary lens: either you’re broken, or you’re fine. But most chronic dysfunctions operate in this middle ground—where a small inefficiency, left unchecked, cascades into widespread symptoms.
CBS and MTHFR SNPs are a classic example. They don’t guarantee disease—but they expose metabolic vulnerabilities that interact with environment, nutrition, and stress. For practitioners using functional medicine and genomics, these are precisely the leverage points we’re trained to find.
So, whether your patient presents with stroke, burnout, PMDD, or panic attacks—consider methylation and sulfur pathways. They may not scream for attention, but they often hold the key.
Final Thoughts: A Case for Rethinking “Rare”
We don’t miss these diagnoses because they’re rare. We miss them because we’re not looking. We let homocysteine drift into the periphery of our workups, despite the fact that it’s a metabolic accelerant for vascular damage, mood instability, and neurodegeneration.
This paper challenges that complacency. It argues—persuasively—that every adult patient with unexplained neurological symptoms deserves a homocysteine level.
Not just for academic curiosity. But because lives, cognition, and independence depend on it.
Reference
Cohen Aubart F, Sedel F, Papo T. Déficit en cystathionine bêta-synthase et déficit en MTHFR chez l’adulte. Rev Neurol (Paris). 2007;163(10):904-910. doi:10.1016/S0035-3787(07)92633-8
Reference
Cohen Aubart F, Sedel F, Papo T. Déficit en cystathionine bêta-synthase et déficit en MTHFR chez l’adulte. Rev Neurol (Paris). 2007;163(10):904-910. doi:10.1016/S0035-3787(07)92633-8