Introduction
Type 2 diabetes mellitus (T2DM), gestational diabetes, metabolic syndrome, obesity, fatty liver disease, and inflammatory bowel disease may seem like distinct clinical entities. But beneath their varied presentations lies a biochemical thread that connects them: homocysteine metabolism.
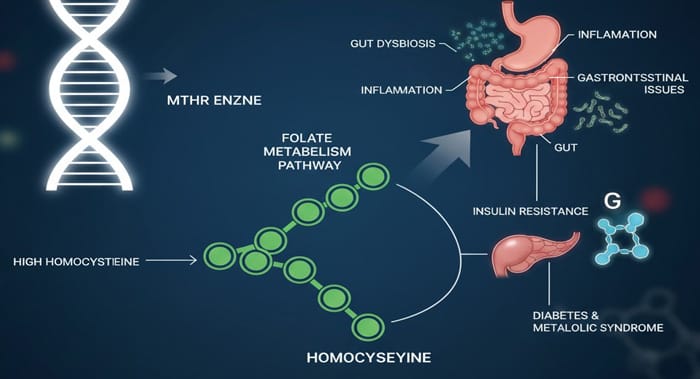
The methylenetetrahydrofolate reductase (MTHFR) enzyme—often discussed in relation to cardiovascular or neurological disease—also plays a surprisingly important role in these metabolic and gastrointestinal conditions. Variants such as MTHFR 677C>T and 1298A>C can elevate homocysteine (HHcy), triggering oxidative stress, impairing insulin signaling, and altering methylation patterns. The downstream effects range from microvascular complications in diabetes to liver fat accumulation and intestinal inflammation.
Let’s take a closer look at how MTHFR polymorphisms weave through these conditions.
Type 2 Diabetes Mellitus (T2DM)
Globally, T2DM affects over 500 million adults, and projections suggest this number will reach 643 million by 2030. Genetic predisposition is one factor driving this epidemic.
MTHFR-related hyperhomocysteinemia has two key mechanistic ties to T2DM:
- Insulin resistance: Homocysteine activates c-Jun N-terminal kinase (JNK), which inhibits insulin receptor substrate-1 (IRS-1). This blunts the insulin signaling cascade.
- Oxidative stress: Homocysteine promotes free radical production and impairs antioxidant enzymes, leaving β-cells—already poor in antioxidant defenses—vulnerable to oxidative injury.
These mechanisms help explain why MTHFR 677C>T and 1298A>C polymorphisms are linked to diabetes risk in some populations.
- Meta-analysis data suggest a strong association between 677C>T and T2DM in Asians, weaker in Caucasians, and absent in some African groups.
- The 1298A>C polymorphism shows even more inconsistency—sometimes increasing risk, other times appearing protective.
Clinical implication: Genetic background and ethnicity matter. A patient’s risk profile isn’t defined by MTHFR alone but by how it interacts with diet, environment, and other genetic variants.
Gestational Diabetes Mellitus (GDM)
Pregnancy imposes a natural state of insulin resistance, driven by placental hormones. For some women, this tips into gestational diabetes.
The role of MTHFR here is less clear-cut:
- Large Chinese meta-analyses point to 677C>T increasing GDM risk, especially in southern populations.
- Smaller Vietnamese and South African studies found no association, though they did note elevated fasting insulin in carriers of the T allele.
Takeaway: MTHFR variants may not directly “cause” GDM but can influence insulin sensitivity, especially when layered on top of the hormonal stress of pregnancy.
Metabolic Syndrome
Metabolic syndrome—characterised by insulin resistance, central obesity, dyslipidaemia, hyperglycaemia, and hypertension—remains one of the strongest predictors of cardiovascular and diabetic outcomes.
Hyperhomocysteinaemia contributes through oxidative stress, cytotoxicity, and impaired nitric oxide signaling. While some studies suggest MTHFR 677C>T increases risk, meta-analyses are inconsistent. Epigenetics—how lifestyle and environment switch genes on and off—likely explain the conflicting data.
Obesity
Here the story gets more interesting. Homocysteine disrupts lipid metabolism through:
- Endoplasmic reticulum stress
- Epigenetic alterations in fat cell biology
Meta-analyses link the 677C>T TT genotype to obesity risk. Yet, paradoxically, some studies show the TT genotype may protect against obesity by lowering cholesterol and triglycerides.
This duality highlights a common theme: genetics rarely dictate destiny in isolation—they interact with diet, exercise, and metabolic context.
Non-Alcoholic Fatty Liver Disease (NAFLD)
NAFLD is now the most common liver disease worldwide. While diet and insulin resistance are central drivers, homocysteine adds fuel to the fire:
- Promoting fat accumulation in hepatocytes
- Inducing endoplasmic reticulum stress
- Damaging endothelial cells within the liver
MTHFR 677C>T has been identified as a global risk factor for NAFLD in some meta-analyses.
Clinical pearl: Even if the polymorphism doesn’t directly predict NAFLD, elevated homocysteine itself—regardless of genetic cause—can be a therapeutic target.
Inflammatory Bowel Disease (IBD)
Chronic gut inflammation in Crohn’s disease (CD) and ulcerative colitis (UC) has genetic, immune, and environmental triggers. While MTHFR isn’t a “core IBD gene,” it contributes via:
- Hypomethylation of pro-inflammatory genes (turning them on)
- Increased neoangiogenesis in the intestinal mucosa
- Impaired antioxidant defenses
The data, again, are mixed. Some populations show no association between MTHFR polymorphisms and UC, while others note that certain genotypes predict disease severity. In Crohn’s disease, 677C>T has been linked to higher risk in specific ethnic cohorts.
Pulling the Threads Together
Across diabetes, obesity, fatty liver, and gut inflammation, one recurring pattern emerges:
- MTHFR polymorphisms → hyperhomocysteinemia
- HHcy → oxidative stress, impaired insulin signaling, hypomethylation
- Result → metabolic dysfunction, vascular injury, inflammation
But the variability across populations underscores a key point: genetic polymorphisms don’t act in isolation. They interact with diet (folate, B12, choline), environment, lifestyle, and epigenetics.
From a clinical standpoint, this means two things:
- Testing homocysteine is often more useful than testing MTHFR alone. It reflects both genetics and lifestyle.
- Targeted interventions—optimising folate/B12 intake, reducing oxidative load, supporting methylation—can reduce risk even in genetically susceptible individuals.
Conclusion
MTHFR polymorphisms offer a fascinating lens into why some patients develop metabolic and gastrointestinal diseases while others don’t. The data remain inconsistent, sometimes contradictory. But the mechanisms—homocysteine-driven oxidative stress and impaired methylation—are robust and biologically plausible.
As with so much in medicine, the answer lies not in single SNPs but in systems biology: how genes, biochemistry, and environment converge. For clinicians, this means approaching MTHFR not as a genetic verdict, but as one modifiable risk factor in the broader metabolic puzzle.
Reference
Araszkiewicz, A. F., Jańczak, K., Wójcik, P., Białecki, B., Kubiak, S., Szczechowski, M., & Januszkiewicz-Lewandowska, D. (2025). MTHFR Gene Polymorphisms: A Single Gene with Wide-Ranging Clinical Implications — A Review. Genes, 16(4), 441. https://doi.org/10.3390/genes16040441